If you’re here reading this, you probably already know that Marquis reagent is one of the most commonly used reagents in the drug testing community. From amphetamines, and substituted-amphetamines (like MDMA/MDA), to natural alkaloids (like mescaline,morphine and codeine) and “bath-salts” ( like methylone and butylone), marquis reagent is known to reliably react with these compounds to produce a specific reaction for each.
The drug testing community has been able to use these reactions to their advantage, but for those of us that are curious, the question is why. Why do these compounds react so reliably with marquis reagent?
First let’s talk some chemistry basics, and than we can get an idea why exactly marquis reagent has founds its place as king of drug testing reagents.
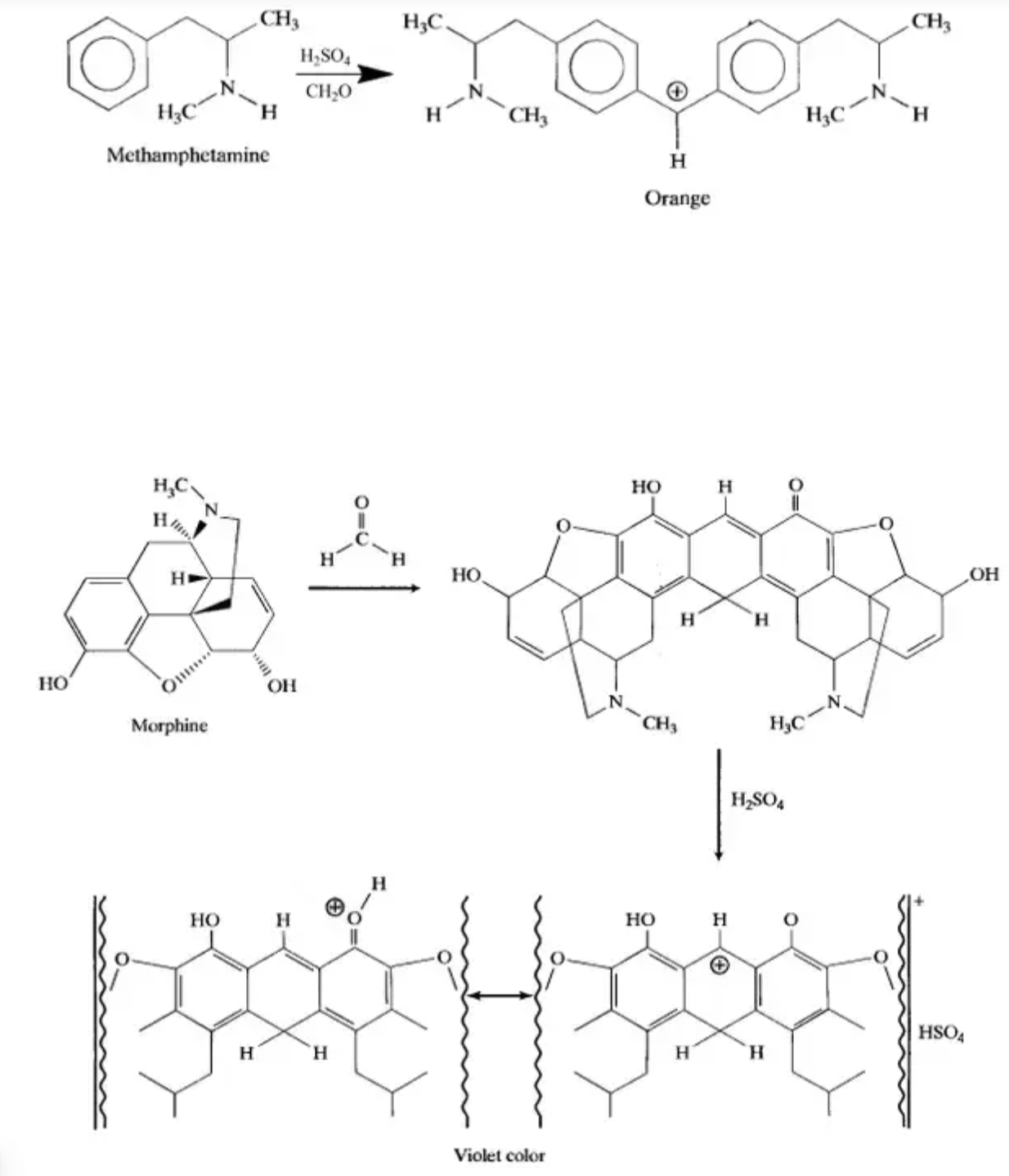
Marquis reagent is a mixture of concentrated sulfuric acid, and formaldehyde. Sulfuric acid is very reactive and with the help of formaldehyde, the mixture catalyzes a reaction that results in the dimerization ( combination of two molecules). So why exactly does the dimerization cause a color to be given off?
Color arises from the way in which colorants interact with light. Colored organic compounds contain groups of atoms whose bonds are unsaturated, such as C=C, C=O, and N=N, which is exactly what marquis reagent creates when it chemically reacts with a given drug (illustration below). The reaction of marquis with a drug creates unsaturated double bonds, which then give off a color when illuminated by light.
Lastly, the common theme with many of the drugs that marquis reagent does react with, is that they contain a nitrogen atom. Many natural drugs are alkaloids (such as morphine, mescaline, codeine), and all alkaloids contain a nitrogen. This fact helps to explain why marquis is such a useful reagent to test drugs.